We have dedicated the last five postings on the role of tyrosine and its metabolism, and how imbalances of this common amino acid may dictate, in part, some of the symptoms related to ADHD.
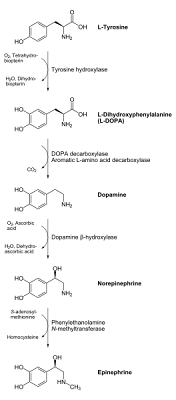
Just for refreshers, here's a diagram of the overall conversion process and metabolism of tyrosine. We have spoken through the first three steps (and the corresponding enzymes and required chemical nutrients) in the process:

Here's a quick recap on our last 5 discussions on ADHD and tyrosine:
Post #1 on ADHD and tyrosine: We examined the overall theory and background behind the use of tyrosine as an ADHD treatment strategy. We saw how it is a chemical precursor to important neurotransmitters (neuro-signaling chemicals responsible for communication among brain cells and the central nervous system) such as dopamine and norepinephrine. We also introduced the concept of the blood-brain barrier, a biochemical barrier which controls the transport of drugs, nutrients and toxins in and out of the brain.
Post #2 on ADHD and tyrosine: here we analyzed the first step of tyrosine metabolism, in which tyrosine is converted to another compound L-DOPA (a common treatment method for Parkinson's patients). This step heavily involves the enzyme tyrosine hydroxylase. However, in order to optimize function of this conversion process, the tyrosine hydroxylase enzyme requires certain vitamins and minerals to act as "co-factors" or "helpers". These include iron, vitamin C, magnesium, zinc, folic acid (namely folate or vitamin B9) and overall adequate antioxidant levels. Secondary nutrients (necessary for enzymes which lead up to the formation of some of the products used by the tyrosine hydroxylase enzyme) include copper, and (as we'll see later on in the tyrosine metabolic pathway), vitamin B12. Deficiencies in one or more of these nutrients could potentially compromise this enzyme's function. Since this first step is actually the slowest (rate-determining) step of the whole tyrosine metabolism process with regards to converting tyrosine to the neurotransmitters dopamine and norepinephrine, making sure we have adequate resources of these "helper" nutrients is crucial to our success.
Post #3 on ADHD and tyrosine: We can essentially bypass this first step of tyrosine to L-DOPA conversion altogether if we just decided to supplement directly with L-DOPA instead. But is L-DOPA more effective than tyrosine as a treatment method for ADHD, or are there some serious drawbacks to this strategy? This third post evaluates and compares both tyrosine and L-DOPA options and compares both their effectiveness as ADHD treatment agents and their comparative safety issues in several different categories.
Post #4 on ADHD and tyrosine: In this post, we examined the second major step of the conversion process in tyrosine metabolism, the conversion of L-DOPA to dopamine. This step requires use of the enzyme DOPA decarboxylase. Like the tyrosine hydroxylase enzyme in the step before it, DOPA decarboxylase also requires nutrient co-factors to optimally function. The main nutrient requirement of this enzyme, however, is a specific form of vitamin B6, known in this case as pyridoxal phosphate. In addition to requiring adequate vitamin B6 levels to function properly, we also saw that other amino acids (namely tryptophan), can actually interfere and even compete with this process, so the post ended with the recommendation to avoid taking in tryptophan-rich foods (which were listed in this fourth post) at the same time as tyrosine was being supplemented.
in post #5 on ADHD and tyrosine supplementation, we examined the conversion process of dopamine to norepinephrine. It is important to note that this process is NOT universal across the body, or even throughout all regions of the brain and central nervous system, for that matter. However, since both dopamine and norepinephrine both can play major roles with regards to ADHD and the symptoms of the disorder, this enzymatic conversion process is still of importance. The enzyme used here for this step of the tyrosine metabolic pathway is called dopamine beta hydroxylase. Interestingly, the gene coding for this enzyme (which goes by the same name, the dopamine beta hydroxylase gene and is located on the ninth human chromosome), has been implicated as a potential hereditary factor for ADHD. Like the aforementioned tyrosine hydroxylase the dopamine beta hydroxylase enzyme is heavily dependent on ascorbic acid (vitamin C) as a cofactor, and heavy utilization of this enzyme (especially without adequate antioxidant pools in place to help regenerate the vitamin) can use up the body's overall supply of vitamin C.
-----------------------------------------------------------------------------------------------
Moving on to our sixth post in our series on ADHD and tyrosine, however, we need to investigate the next step of the process, the conversion of norepinephrine to epinephrine (adrenaline). Keep in mind that this process is not universal, it is dependent on an enzyme called phenylethanolamine methyltransferase, or PNMT for short. Interestingly, the gene which "codes" for this enzyme, also called PNMT, has been linked to a common behavioral sub-component of ADHD called cognitive impulsivity. The PNMT gene is located on the 17th human chromosome.
In contrast to the other main type of ADHD-styled impulsivity, known as aggressive behavioral impulsivity (which is more characterized by arguing, having a short temper, conflicts with peers and adults, and the like, which is more characteristic of oppositional defiant and conduct disorders, and is seen more in the hyperactive/impulsive or combined ADHD subtypes), cognitive impulsivity often has more academic than behavioral inhibitions.
Symptoms of cognitive impulsivity deal more with things such as having trouble waiting in line, struggling with maintaining a continuous focus on school assignments, inability to complete schoolwork, and being prone to every little distraction (a chirping bird outside, the sound of cars passing by on a nearby road, etc.). Cognitive impulsivity is therefore more reflective of the inattentive subtype of ADHD (which is often more frequently seen in girls, and is often more easy to overlook than the other subtypes of ADHD).
It is interesting to note that differences in parent and teacher evaluations often occur over this type of impulsivity, since this type of behavior is often much more visible in a classroom setting. Therefore, if a large discrepancy occurs between the parent and teacher rating scales, which are usually used to help diagnose and assess ADHD, cognitive impulsivity (and possibly even the factor of the PNMT gene) may, in part, be to blame. (Please take this last statement as a possible explanation for this type of behavior and not as an excuse or a "cop-out" for a child's poor performance in school!)
Returning from our aside on the possible genetic relationship between the Phenylethanolamine N-methyltransferase (PNMT) enzyme function and cognitive impulsive ADHD-like behavior, let's return to the chemical process and nutrient requirements of this enzyme. To us visualize this step of the process, here is a chemical depiction of the norepinephrine to epinephrine conversion:
 Even if you're not a chemist, do you see how the norepinephrine molecule added a methyl (CH3) group on to the right end of it to get epinephrine? This is the working of the Phenylethanolamine N-Methyltransferase (PNMT) enzyme.
Even if you're not a chemist, do you see how the norepinephrine molecule added a methyl (CH3) group on to the right end of it to get epinephrine? This is the working of the Phenylethanolamine N-Methyltransferase (PNMT) enzyme.However, the source of this methyl (CH3) group to be added to the molecule needs to come from somewhere. This is where an essential nutrient called S-adenosyl-methionine (as depicted in the diagram above by the downward arrow) comes into play.
S-adenosyl-methionine often goes by other shorter names in the literature and in the grocery aisle, it is often referred to simply as SAMe or just "SAM". We will refer to it as "SAMe" from this point onward.
SAMe is one of the hot new supplements out in the health food aisles these days, and while this blogger personally believes that this nutrient is a bit overhyped, it does offer a number of unique benefits which can possibly cover a whole array of disorders. It is a chemically-modified version of the amino acid methionine. The ability of SAMe to pass on or "donate" a methyl (CH3) group to another molecule (as in the above process where norepinephrine is converted to epinephrine) is a relatively rare property among dietary nutrients, so SAMe does have a number of biochemical implications as a potential supplementation strategy.
As far as psychiatric disorders are concerned, SAMe is a particularly well-known natural supplement for treating depression, and can often have a faster onset than several types of prescription medications (it can also be used in conjunction with antidepressant medications in several cases to augment these medications' effectiveness). SAMe has also been implicated as a potential treatment strategy for other neurological disorders such as Alzheimer's and Parkinson's diseases. However, while anecdotal evidence for SAMe's use in ADHD is moderately strong in some cases, very few reported clinical studies have been done on SAMe for ADHD. One very small study on SAMe and ADHD (only 8 people!) showed relatively positive results, however.
Returning to the diagram here (see below), we see that one of the end products (that's what the curvy arrow means) of this interaction between the PNMT enzyme and the SAMe nutrient is another compound called homocysteine.

We have alluded to this potentially harmful pro-inflammatory compound in some of our previous posts on tyrosine supplementation, and also examined homocysteine in more detail in post further back dealing with ADHD, alcoholism and nutrient deficiencies. As a natural byproduct of this norepinephrine to epinephrine conversion process, we must make sure that we are able to keep levels of homocysteine in check. We will see how we can potentially counter this with B vitamins and other nutrients in our next blog post on ADHD and tyrosine supplementation.
However, the three main points we should take away from this post on tyrosine supplements and ADHD are as follows:
- The conversion process of tyrosine to epinephrine does not occur in all cells, even in the brain and central nervous system. Many regions (even those associated with ADHD) "stop" with dopamine in the overall metabolic process of tyrosine.
- For the brain regions that do accommodate the norepinephrine to epinephrine conversion process, an adequately functioning enzyme called Phenylethanolamine N-Methyltransferase (or PNMT) is required.
- In order for the PNMT enzyme to do its job in converting norepinephrine to epinephrine (adrenaline), adequate supplies of the nutrient S-Adenosyl-methionine (SAMe) are required. This process, however, can leave us with a potentially hazardous byproduct called homocysteine, which must be kept in check to reduce the risk of "inflammatory" diseases such as cancer or cardiovascular disorders. Nutritional intervention strategies must be put in place to help prevent unwanted accumulation of this homocysteine. This is part of the "cleanup process" of the tyrosine supplementation strategy for ADHD, and will be discussed at length in the next blog posting.